SE‑2012
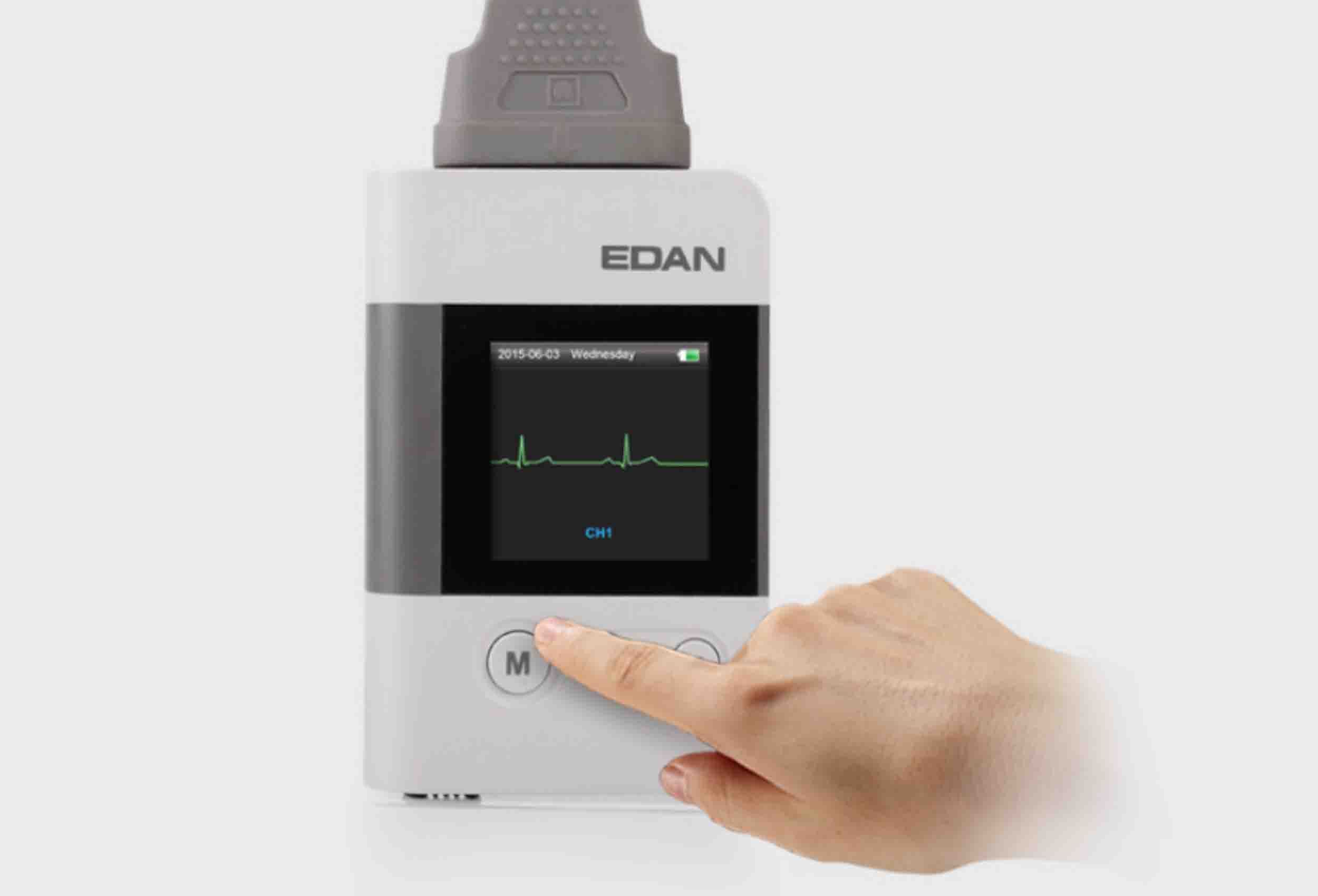
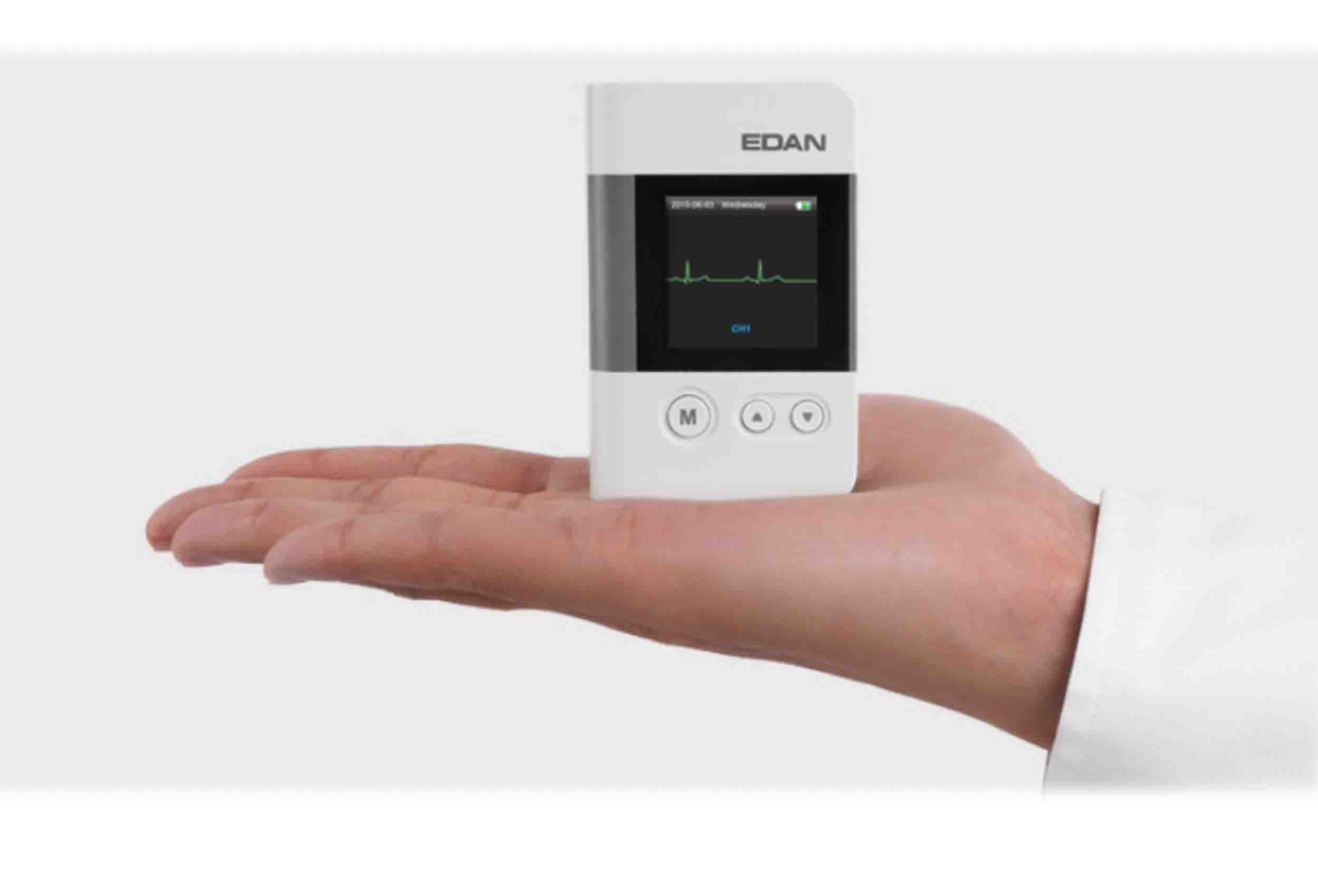
EDAN SE-2012 is a 12-channel Holter ECG system designed for extended ambulatory cardiac monitoring in cardiology practices and hospitals. It combines a lightweight digital recorder with powerful analysis software to help clinicians detect arrhythmias, ischemic changes and other rhythm abnormalities over multiple days of recording.
The compact recorder supports multi-channel ECG acquisition with high-quality signal capture and allows lead-check verification at hook-up. Data are stored on removable memory and later transferred to the analysis workstation. The Holter software provides full disclosure review, automatic beat detection, arrhythmia and ST-segment analysis, as well as heart rate variability and trend reports. With flexible protocols and configurable analysis templates, SE-2012 supports efficient interpretation and reporting in busy cardiac clinics.
- 12-channel Holter ECG recording
- up to 7 days continuous recording (depending on configuration)
- Lightweight recorder design (approx. 60 g) for patient comfort
- Compact dimensions (around 75 × 50 × 15 mm)
- On-device display for lead check and status: OLED or LCD trend/lead check display on recorder
- Recording media: Removable SD/microSD card storage
- Power: Single AA or AAA battery (model dependent)
- Full disclosure review, beat classification, arrhythmia analysis, ST analysis, HRV analysis, AF analysis, HR turbulence and trends
- Automatic and manual edit tools for ECG data
- Configurable analysis templates and reporting formats
- Provides multi-day, multi-channel Holter ECG data for comprehensive rhythm assessment
- Lightweight recorder improves patient compliance and comfort
- High-quality signal capture supports accurate arrhythmia detection
- Advanced analysis software shortens review time and supports detailed reporting
- Flexible protocols cover a wide range of diagnostic Holter indications
Ambulatory Holter ECG monitoring for arrhythmia detection, atrial fibrillation screening, ischemia assessment, syncope investigation and long-term cardiac rhythm evaluation.
Manufactured under ISO 13485 quality management system. CE-marked Holter ECG device.

